CONffIDENCE WP2a: Coccidiostats
Objectives
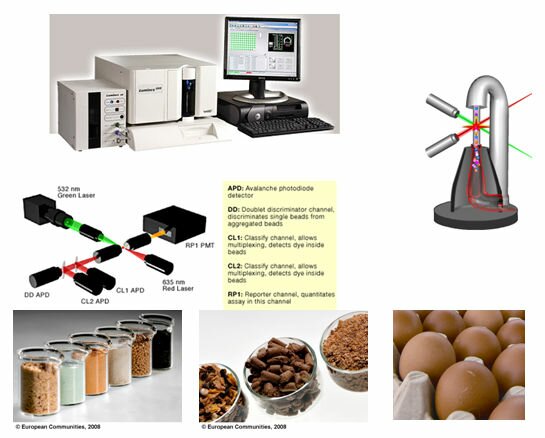
• Development and validation of a flow cytometry based multiplex immunoassay for residues of lasalocid A, monensin, salinomycin, narasin, and nicarbazin in eggs and their cross-contamination in non-targeted feed (laying hens feed),
- Simplified sample preparation procedures for eggs and feed,
- in-house validation,
- training,
- small-scale inter-laboratory study and comparison of performance characteristics with existing methods
• Impact demonstration: carry-over study of lasalocid from laying hens feed to eggs aiming at contribution to a predictive hazard behaviour model
Overview
Coccidiostats constitute the main choice to fight coccidiosis, a major parasitic disease in poultry. Recently, the Commission requested an opinion of EFSA on the risks involved for animal and public health as a consequence of unavoidable cross-contamination of frequently used coccidiostats authorised as feed additive into non-target feeds and consequently the presence of such residues in food of animal origin.
The Commission also requested representatives of the industry to provide factual information on cross-contamination in feed for non-target species. Hence this project will address the priority coccidiostats lasalocid, monensin, narasin, nicarbazin and salinomycin in laying hens feed produced on the same production line as broiler feed containing the coccidiostats at the normal therapeutic dose, and consider both eggs and feed as sample matrices of interest.
Method development will concern the simultaneous detection of residues of selected coccidiostats in eggs and in non-targeted feeds in the frame of a potential cross-contamination, from feed produced with the highest authorised dose of the coccidiostats (e.g. broiler feed) into the afterwards produced non-targeted feed (e.g laying hens feed), taking into account hypothetical carry-over rates of 2 %, 5% and 10%. To this end flow cytometry based multiplex immunoassay prototype test kits will be developed using the Luminex TM platform.
The main components related to method validation in this project are (i) the preparation of test materials, (ii) the in-house validation of the developed multiplex assay and (iii) the organisation of a small-scale interlab study within the consortium. Finally, a carry-over study will be carried out for lasalocid using four groups of laying hens from 30 to 55 weeks. One group is the control group; three other groups will be fed with laying hen-feed containing, respectively, the normal therapeutic dose (90-125 mg of active substance per kg of complete feed as laid down in (EC) No 2037/2005, 2.5% of the normal therapeutic dose and 10% of the normal therapeutic dose. The assessment of the carry-over of lasalocid from feed to eggs will then be carried out using the developed and validated flow cytometry based multiplex immunoassay.
Work Package leader
Dr Ursula Vincent (European Commission, Joint Research Centre, Institute for Reference Materials and Measurements, EC-JRC-IRMM, BE)
 Ursula Vincent is a senior scientist in the Food Safety and Quality Unit at JRC-IRMM. She has more than 10 years experience in development and validation of analytical methods. Currently, she is supervising and coordinating projects related to contaminants and residues analysis in food and feed matrices as the project leader of the Community Reference Laboratory for Feed Additives (Control).
Ursula Vincent is a senior scientist in the Food Safety and Quality Unit at JRC-IRMM. She has more than 10 years experience in development and validation of analytical methods. Currently, she is supervising and coordinating projects related to contaminants and residues analysis in food and feed matrices as the project leader of the Community Reference Laboratory for Feed Additives (Control).
Work Package deputy leader
Dr. Philippe Delahaut, (Hormonology Department – CER Groupe, BE)
 Philippe Delahaut is a senior scientist and the director of the Hormonology department at the CER groupe in Belgium. He has 20 years of experience in food analysis and development of immunological methods. He is in charge of the management of animal facilities for the production of antibodies and an expert at Belgian (AFSCA) and European (CEN/TEC 275/WG 12 – Food allergens) committees.
Philippe Delahaut is a senior scientist and the director of the Hormonology department at the CER groupe in Belgium. He has 20 years of experience in food analysis and development of immunological methods. He is in charge of the management of animal facilities for the production of antibodies and an expert at Belgian (AFSCA) and European (CEN/TEC 275/WG 12 – Food allergens) committees.
For further information about the work package 2a, please contact
